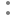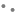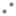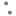Fig. 3: Lineweaver-Burk plots for the kinetic analysis of xanthine oxidase activity inhibited by A. crassna.
The (♦), (•), (Δ) represent A. crassna at concentration of 0.5, 1.5 and 4.5 mg/ml, respectively.

Fig. 4: Effect of the water extract of A. crassna leaves on the plasma uric acid concentration on potassium oxonated-induced hyperuricemic mice.
Data are expressed as mean±SEM (n=6). Group 1 represents normal control group, group 2 means untreated hyperuricemic mice, and groups 3, 4, 5 and 6 represent potassium oxonate-induced hyperuricemic mice treated with allopurinol (5 mg/kg), 500, 1000, and 3000 mg/kg A. crassna, respectively. *indicates a statistically significant difference at P<0.05, when compared with group 2.
3000 mg/kg, as compared with hyperuricemic control group (P<0.05). However, significant differences were not observed between each dose of treatment groups.
The liver homogenate of mice treated with A. crassna extract at the dose of 1500 and 3000 mg/kg significantly inhibited the formation of uric acid by inhibiting XOD activity about 47.2% and 63.6%, as compared with the untreated hyperuricemic group (P<0.05, respectively) as shown in Table 1. Allopurinol markedly inhibited XOD activity by about 81.3% (P<0.05).
Several bioactive compounds found in the aqueous extract of A. crassna leaves have been identified such as fluorofenone 3-C-β glucose, epicatechin gallate, epigallocatechin gallate (EGCG), mangiferin[3]. Previous studies supported that mangifenin and
|
TABLE 1: EFFECT OF THE AQUEOUS EXTRACT OF A. CRASSNA LEAVES ON RESIDUAL ACTIVITY OF XOD IN LIVER
| Groups |
XOD activity
(nanomole/min/mg protein) |
XOD Inhibition (%)
(compared with group 2) |
1
2
3
4
5
6 |
0.99±0.09
2.14±0.28
0.40±0.08*,#
1.7±0.51
1.13±0.22*
0.78±0.09** |
–
–
81.3
20.5
47.2
63.6 |
Data are mean±SEM, obtained from 6 mice per group. Group 1 represents normal control group, group 2 is untreated hyperuricemic mice, and groups 3, 4, 5 and 6 represent potassium oxonate-induced hyperuricemic mice treated with allopurinol (5 mg/kg), 500, 1,000 and 3,000 mg/kg A. crassna, respectively.# represent P< 0.05, compared with group 1 and * represent P<0.05, compared with group 2
EGCG expressed a hypouricemic effect. However, the XOD inhibitory effect of EGCG was quite low with inhibition less than 5%[12]. Mangiferin was detected in the highest level among compounds found in the extract (1.44%). A previous study found that mangiferin had a potent hypouricemic effect associated with inhibiting the activity of xanthine dehydrogenase and XOD[5,6]. Therefore, we employed mangifenin as the standardized marker of the extract and thought that it may contribute for the antihyperuricemic effect of the extract in the present study.
Inhibition of XOD is one of the drug targets for the treatment of hyperuricemia. In vitro study suggested that A. crassna extract inhibited the XOD activity in a concentration-dependent manner with IC50 more than 1 mg/ml, indication of very weak XOD inhibitory effect. Oral administration of 3000 mg/kg of A. crassna extract significantly prevented the increase in plasma uric acid level induced by potassium oxonate in mice and distributed into the liver, and could inhibit the XOD activity in the liver, however, there were no significant differences in plasma uric acid concentration after administration of each dose of A. crassna extract (500, 1000, 3000 mg/kg). Taken together, it can be concluded that effect of A. crassna on XOD inhibition was negligible.
From our knowledge, there are two mechanisms to reduce plasma uric acid concentration. First mechanism is to inhibit XOD enzyme and the latter is to inhibit the reabsorption of urate in renal i.e. renal mRNA and protein levels of urate transporter 1 (URAT1), glucose transporter 9 (GLUT9), organic anion transporter 1 (OAT1) and organic cation/carnitine transporters
(OCT1/2, OCTN1/2). Administrations of A. crassna extract showed possibility to decrease plasma uric acid concentration, although inhibitory on XOD might not be involved. The inhibitory effect on renal urate reabsorption of mangiferin, a major bioactive compound found in A. crassna, has been reported[6].
Acute toxicity test of A. crassna extract in mice showed no sign of toxicity or death at the doses up to 15000 mg/kg body weight[13]. In practice, thedose of A. crassna extract recommended commercially in 10 mg of tea with 500 ml of hot water[14] is much lower than the effective doses observed in the present study. We conclude from the results obtained from the present study that A. crassna leaf extract could not be used as alternative medicine for treating of acute hyperuricemia and the study of long term administration on urate reabsorption effect is needed.
Acknowledgements:
We would like to deeply thank Prof. Gary H. Smith, Department of Pharmacy Practice and Science, University of Arizona, College of Pharmacy, Tucson, AZ for kindly reviewing our manuscript.
Financial support and sponsorship:
This work was financial supported by School of Pharmacy, Showa University, Japan.
Conflicts of interest:
There are no conflicts of interest.
|